Recently, the USP (United States Pharmacopoeia) published an article in the Journal of Natural Products titled, “Cannabis Inflorescence for Medical Purposes: USP Considerations for Quality Attributes”. The article conclusively states that qPCR is the most sensitive method for Aspergillus testing.
This published scientific work was developed by a panel of experts to provide information and guidance to state cannabis regulators and laboratories who are charged with addressing the public health risks associated with medically used cannabis. Given the wide variance among state and even country regulations, this article could not be more timely in providing much-needed consistency about the core quality attributes and tools to administer quality control of this plant and its uses. Here are the five most important takeaways with regards to microbial testing:
- Aspergillus contamination is of “particular concern” especially when consumed by patients who are immunocompromised.
- qPCR is “by far the most sensitive” method for detecting pathogenic species of Aspergillus.
- Enrichment is NOT optional when testing for Aspergillus contamination.
- The USP’s recommendations and findings have significant regulatory implications.
- If your lab isn’t using qPCR now, it most certainly will be.
We couldn’t agree more with the USP’s conclusions when it comes to Aspergillus testing. Not because we happen to offer Aspergillus qPCR assays, but because as scientists and researchers, we long ago came to the same conclusion. That’s why we selected qPCR technology from the beginning and call for an enrichment period in our protocol. As qPCR champions ourselves, we applaud the USP’s efforts to clarify the debate about which testing method is best, and we wholeheartedly support their efforts to educate the many different stakeholders who must understand why this conclusion is the right one.
Aspergillus Testing Must be a Part of Every Serious Testing Regimen
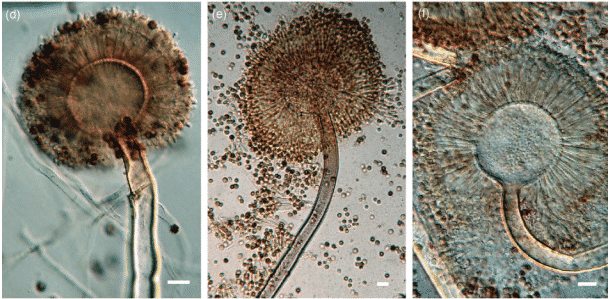
The article and the USP’s accompanying statement identify the four pathogenic species of Aspergillus (A. flavus, A. fumigatus, A. niger, and A. terreus) as a particular concern for patients using cannabis, as they can cause potentially deadly infections. This is important because the USP’s previous cannabis testing guidance did not mention Aspergillus.
The authors point out the inadequacies of using plating to detect Aspergillus, citing that pathogenic species are often indistinguishable from their non-harmful relatives. Although not mentioned in the article, Aspergillus is also an endophyte, which makes it difficult to detect with plating methods because the plant cells are not lysed to access the pathogens living inside.
The USP article did state that the enrichment of cannabis samples is essential for Aspergillus testing. This settles a long-running debate, typically marked by misinformation, about whether sample enrichment can be skipped or replaced with a spin step to speed up the testing process. According to the USP, enrichment is essential to best practice.
Lastly, the USP article states that there is no compendial method for Aspergillus testing, but the AOAC is currently evaluating the various methods that are available. We are proud to report our PathoSEEK Aspergillus assays are among those methods, and we expect to receive approval once the process is complete.
UPDATE: In August 2021, The AOAC PTM Program certified the PathoSEEK® 5-Color Aspergillus Multiplex Assays using the SenSATIVAx® Extraction Kits for detection of Aspergillus in dried cannabis flower and infused edibles.
Why a Peer-Reviewed Paper and not a Monograph
This article was presented as a scientific paper that cited two members of the USP as authors, Michael Eisenstein, M.S., USP Science Writers and Editor, and Kristi L. Muldoon, Ph.D., USP’s Director of Regulatory Science. The paper was not presented as an official monograph because of cannabis’ current federal status of illegality. Here is the USP’s formal statement regarding the distinction and explaining the reasons for their decision.
“The USP is a scientific nonprofit organization that plays a leading role in developing and formalizing standards for medicines, dietary supplements, and foods based on expert advice from the medical and scientific community. USP standards are enforced by FDA in the U.S. In recognition of the fact that the U.S. federal government, including FDA, does not recognize cannabis as a legal drug product, USP has decided not to create formal compendial standards at this time. As an alternative, USP has opted to publish the findings of its experts in the form of a scientific paper rather than a conventional USP monograph, while still employing a rigorous process similar to the one typically used by the organization to formulate its quality standards.”
A Blueprint for Regulatory Clarity and Consistency
The scientific community sees this paper as so significant, there have been calls to adopt its recommendations on cannabis testing as regulatory guidelines for the new states coming into the legal markets, and even whole countries to rethink their regulatory stance. In that regard, Robin Marles, Senior Scientific Advisor at Health Canada’s Food Directorate and Chair of the Botanical Dietary Supplements and Herbal Medicines Expert Committee of USP, said, “The recommendations offered in the Journal of Natural Products article provide a valuable foundation for alignment of testing and quality of products derived from cannabis, and…the Canadian government may consider these guidelines as legal requirements for medical cannabis.”
Canada isn’t the only jurisdiction considering stricter standards for cannabis testing. Every newly legalized state needs expert guidance about how their regulations should be structured and why. Plus, there are states like Washington, Oregon, and Colorado, where the majority of their labs are still using plating, which fails to differentiate between good and bad microbes. The labs using these methods are very likely failing a great many samples that should be passed and passing some that should be discarded. Furthermore, culturing for yeast and mold fails to detect dead organisms, which can still be harmful if inhaled or enriched.
The Future of Cannabis Testing. Brought to You by qPCR
If you’re not already using the most effective, accurate testing method available, as defined by the USP, perhaps you should be, and sooner rather than later. The cannabis industry is growing every day in surprising and consequential ways on its way to fulfilling its destiny as the world’s most profitable agricultural crop and leading source of pharmaceuticals for the 21st century. In that not-too-distant future, regulators, patients, clinicians, growers and yes, laboratories, will demand nothing less the highest quality products. You’ll need to be ready.
That fact that states have disparate regulations is not a sign that qPCR is ineffective or impractical; it’s a symptom of the industry’s immaturity and lack of consistent regulatory oversight state-to-state. Hopefully, the USP article will be a much-needed step towards establishing nationwide cannabis testing standards that include Aspergillus.
The competitive and commercial environment is already changing, and not a moment too soon. More labs are adopting qPCR to separate themselves from less conscientious competitors, and quite frankly, to protect themselves from the potentially ruinous consequences of having passed a batch of contaminated cannabis that could make a patient very sick, or most tragically, kill them.
After Michigan regulators added Aspergillus testing to the regulations, every lab in the state adopted qPCR. Rose City Laboratories in Portland also recently became the first lab in Oregon to adopt qPCR, and we expect more to follow.
We encourage you to read the full USP article and the accompanying statement for yourself. We also encourage you to visit our Aspergillus page, which has more information about the risks of Aspergillus contamination on cannabis and data we have generated to demonstrate qPCR is the best method for testing. And be sure to contact us when you are ready to add Aspergillus testing to your lab.