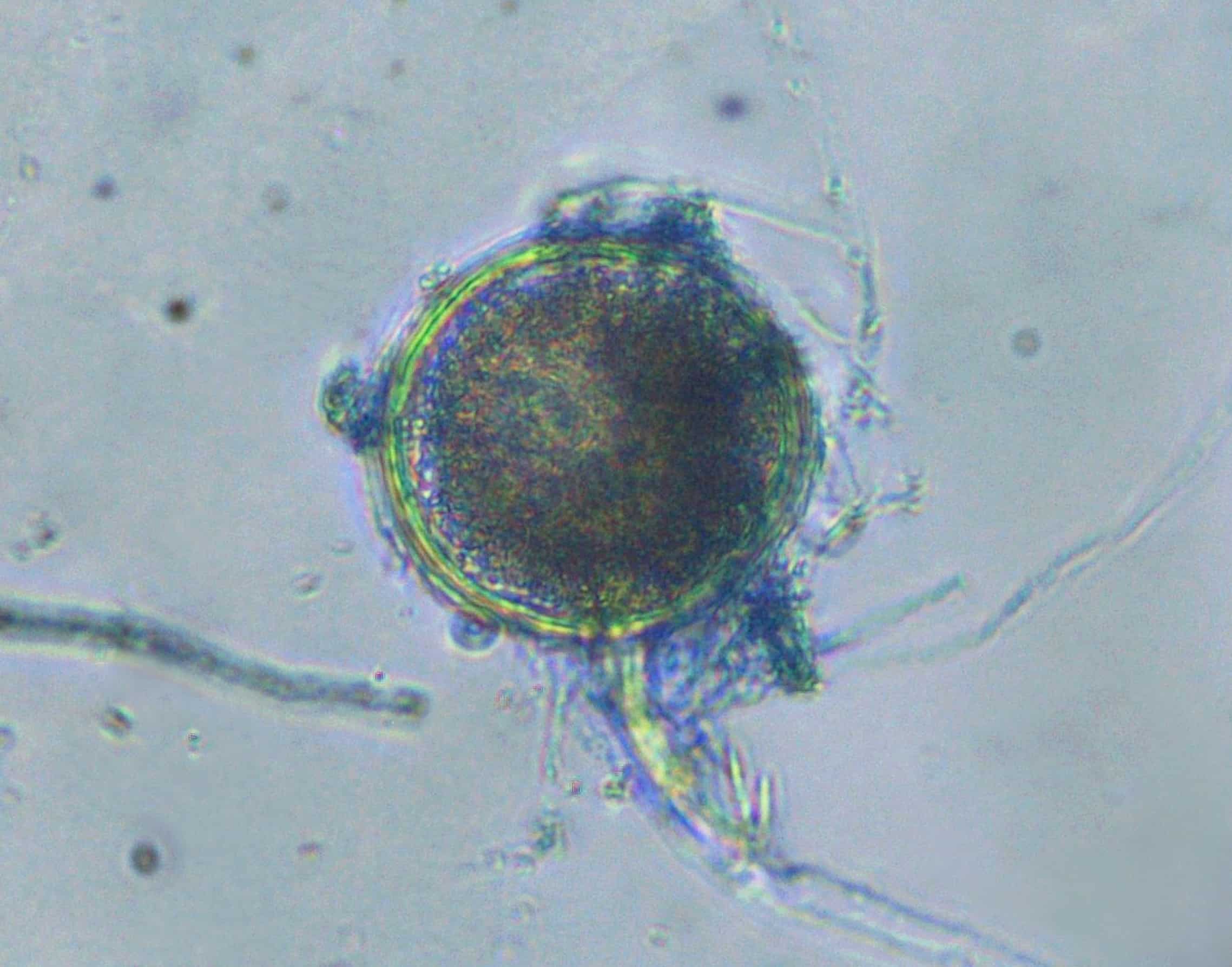
On February 7, the Daily Mail reported a cancer patient in northern California died from a fungal infection that authorities suspect was caused by the inhalation of contaminated medical cannabis.
This tragic result, unfortunately, comes as no surprise to our team at Medicinal Genomics. Since 2015, Medicinal Genomics has been studying the spectrum of microbes that can be found on medicinal Cannabis. As a result of this research, our company has published two papers warning the community that the widely-used method for detecting microbial contamination on cannabis, petri dish plating, is dangerously inaccurate and fails to detect some of the most harmful microbes, such as Aspergillus. Our papers can be found here:
- Cannabis microbiome sequencing reveals several mycotoxic fungi native to dispensary grade Cannabis flowers
- Metagenomic analysis medicinal Cannabis samples; pathogenic bacteria, toxigenic fungi, and beneficial microbes grow in culture-based yeast and mold tests
These papers have also shown that quantitative PCR is a superior method for detecting microbes on cannabis, and unlike the current methods in use, the qPCR methods have been validated for use on the cannabis plant. The cannabis industry, however, has been slow to adopt qPCR, which is the gold standard for such type of testing in the food and agriculture industries. The rate limiting factor for qPCR adoption in the cannabis testing market, as we have been told by cannabis testing laboratories across the country, is the incremental cost per test (~ $10) to run qPCR versus the cost of plating on petri dishes. From a health, safety, and economics perspective, this approach is illogical. Cannabis sells for ~$1,000 per pound compared to ~$2 per pound for other agricultural products on which qPCR is used regularly to ensure product quality. Until the standards for microbial detection on cannabis change, patients will continue to be at risk for microbial infections.
We would like to applaud Dr. George Thompson for recently publishing a similar peer-reviewed manuscript using DNA-based methods to ascertain microbial risks and bringing more attention to this regulatory issue.
Furthermore, molecular techniques can be used to assess whether this cancer patient’s infection was actually cannabis derived. This is possible by using PCR and sequencing as performed by Remington et al. on the cannabis material and on the patient to confirm such an event.
Rather than jumping to conclusions from a news story about cannabis contamination (which may in fact be the case), officials should confirm, via molecular methods, that a fatal infection occurred from the consumption of contaminated Cannabis or from another source, such as a hospital acquired infection. Once confirmed, the scientific data can help drive the appropriate regulations forward to ensure patient safety. Unfortunately, most regulations passed to date for microbial detection do not appropriately address patient safety and often suggest the use of antiquated, inaccurate technologies. For instance, we have peer-reviewed evidence that the currently accepted 48-hour Petrifilm-based method currently in use fails to detect some of the most harmful microbes found on cannabis. The State of Colorado has recently come to similar conclusions and has moved their Petrifilm detection times from 48 hours to 60-72 hours while referencing a paper suggesting 120 hours may be required. And even with these adjustments to the regulations, Petrifilms will never give as accurate results as PCR.
All technologies used to ensure product quality and patient safety should be peer reviewed. DNA-based methods are imperative to patient safety, as they are accepted, peer reviewed, and have been used for decades in other industries for similar purposes.
Kits to perform qPCR-based microbial testing on cannabis are commercially available at medicinalgenomics.com. We hold the largest sequence database of microbes found on cannabis and have kits that perform these tests in hours as opposed to days.
The technology exists to ensure safer cannabis for patients. Let’s make measured changes before another patient is harmed while demanding peer reviewed science is used to guide the regulatory process. In an era of fake news, science by press release with “beliefs” derived from companies that have a vested interest in seeing more cannabis safety testing should be hyper scrutinized. This extends to our own work at Medicinal Genomics and underscores our publication history in this space.