The cannabis industry has a problem…
Culture-based testing is the industry standard for detecting harmful microbes on cannabis flower, but the most widely used culture-based tests have never been validated for use on cannabis.
Our team used genetic sequencing technology to evaluate the accuracy of culture-based microbial tests on cannabis flower, and our findings were peer reviewed and published on F1000.
Read our Microbial Testing Study
Our results show that culture-based tests do a poor job of measuring the level of microbial contamination on a cannabis flower, more specifically:
- Culture-based yeast and mold tests do not effectively discourage bacterial growth, which can lead to inappropriate failures.
- Significant levels of Clostridium botulinum (botulism) were observed in two-thirds of the samples after incubation in the hermetically sealed cards of the Biomerieux Total Yeast & Mold platform.
- Beneficial microbes, which are known to reduce the use of fungicides, grow very well in culture mediums, which can lead to inappropriate failures.
- Aspergillus—the only microbe to ever be associated with clinical harm concerning cannabis—grows poorly in culture mediums, and is therefore severely under-reported by current culture-based platforms.
The shortcomings we have observed in culture-based tests represent a serious concern to cannabis patients, many of who are immunocompromised. The summary below will further explain the shortcomings we observed.
What’s growing in that dish?
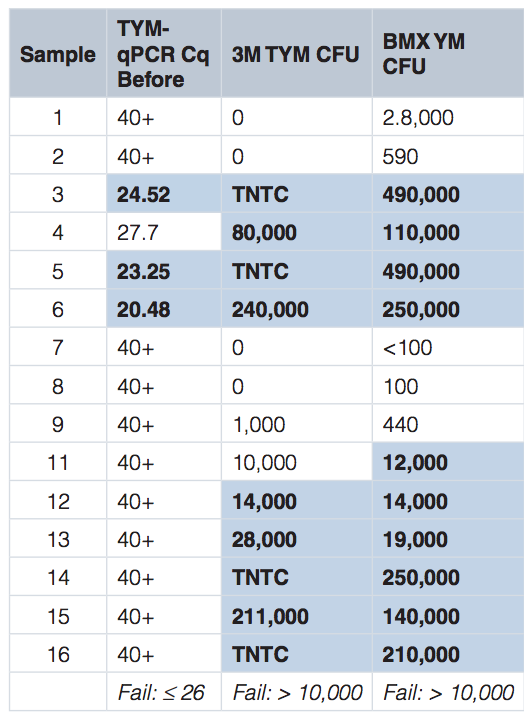
Our team obtained 15 cannabis samples, that had failed culture-based microbial testing, from 7 different growers and tested them on the Medicinal Genomics’ PathoSEEK™ Microbial Safety Testing Platform, as well as the two most popular culture-based tests, 3M and BioMerieux (BMX). In the table below, the highlighted cells indicate a failure. As you can see, qPCR failed 3 samples, 3M failed 9, and BMX failed 10.
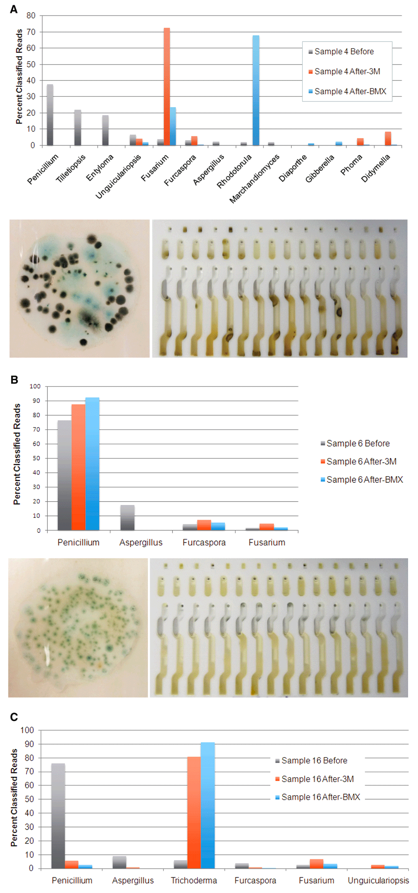
To better understand the discordance of the results, we used genetic sequencing to identify the DNA of the microbes living on each sample before and after culturing.
We found that several bacteria species grew very well in the total yeast and mold cultures, even though antibiotics were present to discourage their growth. The table below shows several bacteria species, including Clostridium botulinum (botulism), that were detected in low levels prior to culturing and were much higher after culturing. Since culture-based tests can’t distinguish between fungi and bacteria colonies, all colonies that grow in the culture are counted, and these off-target bacteria colonies can trigger false positives.
Furthermore, we found that the fungi species that were present after culturing bear little or no resemblance to what was found on the flower prior to culturing. The table below shows the microbial composition of three cannabis samples before and after two different types of culture tests. As you can see, certain fungi species grew very well in the culture, while others do not grow at all.
These results show that what grows in a culture medium is not representative of the microbial composition on the cannabis flower and may lead to false positive results. The 3M and Biomerieux tests were not designed to be used on cannabis and do not take into account the plant’s own microbiome, a unique combination of beneficial microbes, fertilizer, and pesticides, which can influence what grows in the culture.
False positive test results are a concern for cannabis patients because cultivators will often treat failed cannabis with Myclobutanil (also known as Eagle 20). More research needs to be done on the effect Myclobutanil can have on humans when burned and inhaled. It is important to note, that Myclobutanil is not approved for use on tobacco.
Where is the Aspergillus?
Aspergillus is arguably the most significant fungal threat to patients who use medical cannabis. Aspergillosis has been reported in numerous immunocompromised patients and, to date accounts for the only clinical reports of fatalities associated with an infectious organism linked to cannabis consumption.
What is particularly concerning from our results is that Aspergillus grows very poorly in the 3M and Biomerieux cultures. Although Aspergillus species were present in 15 plant samples before culturing, they were only detected at low levels in 3 samples after culturing on either 3M or BMX.
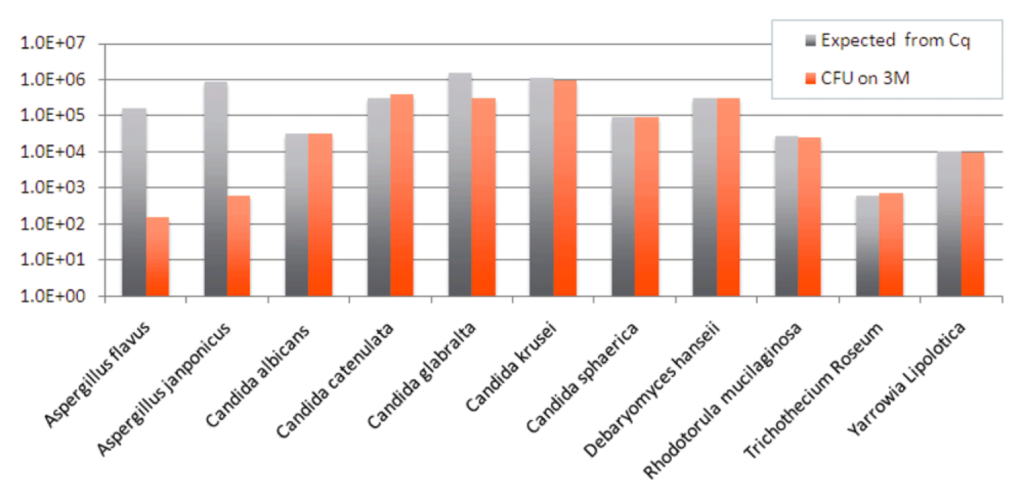
The earlier graph shows the microbial composition of three samples where our qPCR test detected Aspergillus DNA on the cannabis flower sample prior to culture-based testing. In two samples, our qPCR found no Aspergillus DNA in either culture medium following incubation. In the other, a small amount of Aspergillus DNA was detected in the 3M plate.
To further investigate how well Aspergillus grows in the 3M Rapid TYM Petri-Films, we plated 10 fungal monocultures from ATCC stocks and measured to see if what grew in the culture matched the expected amount, based on qPCR testing. As you can see, most of the species grew as expected, but the Aspergillus species grew less than half the expected amount.
What does this all mean?
Culture-based testing methods do not accurately measure the microbes present on a cannabis sample.
The microbiome in the plant material tested changes radically after culturing, such that the microbes and counts that are finally observed bear little or no resemblance to those of the starting sample. This is a serious issue, which clearly has implications beyond Cannabis safety testing. The 3M and BMX platforms tested here are also used widely in the food testing industry.
Perhaps the most concerning observation is that Aspergillus – the only microbe to ever be associated with clinical harm concerning cannabis – grows poorly, and is therefore severely under-reported by current culture-based platforms. The differential growth of other toxigenic fungi, depending on the companion species present, further influences the results. Bacterial pathogens are not uncommon, and beneficial bacteria are also capable of influencing the growth or inhibition of other flora.
Our team demonstrated that qPCR testing is capable of accurately quantifying and identifying a wide spectrum of microorganisms present on Cannabis samples, while avoiding false positives due to the presence of bacteria for fungal testing. qPCR testing is rapid and is capable of distinguishing between harmful and beneficial microbes – permitting the use of the latter in organic cultivation practices to eliminate the need for reliance on chemical fungicides.