Within the past 20 years, medical literature has highlighted the growing evidence supporting the association between epilepsy and genetics. Numerous publications have highlighted linkages between single gene mutations and specific syndromes (e.g., SCN1A and Dravet Syndrome) to increasingly complex polygenic associated epilepsies.
Sequencing of patients with SMEI (severe myoclonic epilepsy in infancy) requires diverse genomics expertise, as there are hundreds of genes thought to be involved in epilepsy, many of which are highly variable ion channel genes. Furthermore, the subtle nuances of mutations in the genes associated with epilepsy require a sophisticated detection and bioinformatics analysis approach.
There are many publications that have helped increase the understanding of epilepsy1-5, including the work done by Lemke et al (250 genes across 50 people), the Epi4K project (Exomes across 264 patients) and Carvill et al (65 genes across 500 patients).
Courtagen’s epiSEEK® comprehensive sequence analysis test provides extensive genetic analysis and clinical interpretation of data generated by the complete sequencing of all genes known to be associated with epileptic and seizure disorder phenotypes. The updated panel includes several recently published genes as well as genes for other categories of disorders including inborn errors of metabolism, congenital disorders of glycosylation, peroxisomal biogenesis disorders, seizures related to intellectual disability, and drug metabolism (cytochrome P450 genes and genes in the cannabinoid and cannabadiol pathways).
Knowing the genetic basis of a patient’s epilepsy can be valuable for obtaining a definitive diagnosis, estimating prognosis, determining recurrence risks, and guiding treatment choices. In many cases, the precise genetic diagnosis can be important in therapy selection, particularly when there are known contra-indications or recommended treatment options based on genetic results.
Most clinical tests only survey 100 genes with 50-100bp reads and fail to detect insertion or deletions larger than 5bp. To rectify this clinical blind spot, additional assays, such as MLPA, are typically run on the sample. These tests, however, do not detect variants smaller than 150bp. It is important to understand that there are 10X more 5-150bp size indels in the clinical databases than there are indels of 150bp in size or greater. Using paired 250bp reads, we can detect up to 50bp indels and expand the clinical sensitivity of our test as described in this document.
Why is sequencing important?
There are a couple dozen different Anti-Epileptic Drugs (AEDs), some of which have known countra-indications based on ones genetic profile. Often, patients are on multiple AEDs at once, experiencing varying degrees of success. With the technology advances in sequencing, more information is available to understand the drug metabolism profiles of specific genotypes to AEDs. See reference information below:
Cannabidiol (CBD) is one of over 85 compounds found in cannabis that belong to a class of molecules called cannabinoids. Of these compounds, CBD and tetrahydrocannabinol (THC) are usually present in the highest concentrations, and are therefore the most recognized and studied. It is a major phytocannabinoid, accounting for up to 40% of the plant’s extract. CBD is considered to have a wider scope of medical applications than THC. CBD and THC levels tend to vary among different plants. Marijuana grown for recreational purposes often contains more THC than CBD. Unlike THC, CBD does not cause a high. While this makes CBD a poor choice for recreational users, it gives the chemical a significant advantage as a medicine, since health professionals prefer treatments with minimal side effects.
CBD is non-psychoactive because it does not act on the same pathways as THC. These pathways, called CB1 receptors, are highly concentrated in the brain and are responsible for the mind-altering effects of THC.
A 2011 review published in Current Drug Safety concludes that CBD “does not interfere with several psychomotor and psychological functions.” The authors add that several studies suggest that CBD is “well tolerated and safe” even at high doses.
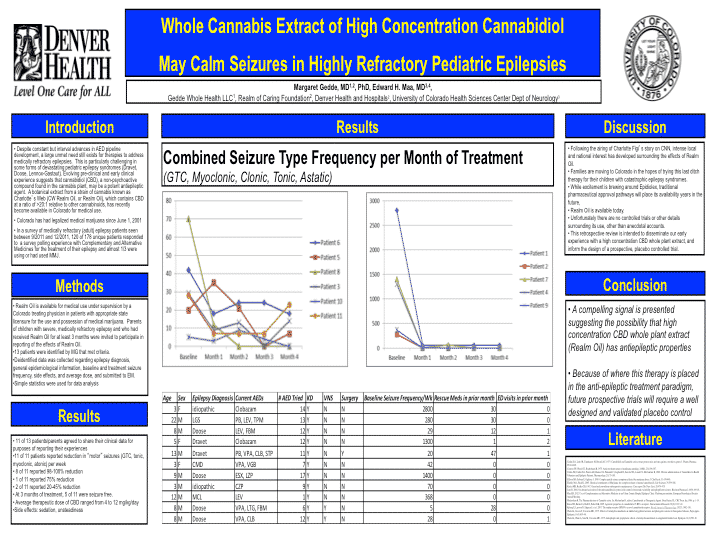
Denver Health recently completed a study on Dravets and CBD. This is the best study surveying parents with children using CBD for refractory epilepsy (Porter and Jacobson. cbd epilepsy 4095091). In this study 84% (16/19) of the patients reported some reduction in seizure frequency, with 42% reporting a 80% reduction in seizure frequency, and with 2 patients being 100% seizure free. Dr. Edward Maa and Dr. Margret Gedde presented very promising information at AES 2013.
Most Dravet’s patients have missense mutations in the SCN1A gene (sodium channel). GW Pharma has published work demonstrating CBD’s lack of affinity for sodium channels. There is prolific literature on cannabidiol impacting calcium regulation via PPARgamma. Listed below are are few links:
It’s possible this interaction is through Calmodulin which is known to interact with SCN1A.
There is also research that supports the variable expressivity of Dravet Syndrome is due to other modifier genes. Listed below are papers showcasing CACNA1A, a gene encoding a calcium channel, mutations modifying the SCN1A mutations.
Clinical trial reviews on CBD are fairly neutral to skeptical over the design of previous CBD trials. The toxicity is reported to be very low and well tolerated, but the double blinding and placebo controls are often lacking.
There has also been a reported case of Sevoflurane contra-indication with CBD. Patients using Sevoflurane based anethesia usually regain consciousness in 25 minutes after the gas is removed. In one case, a patient who was on CBD regained consciousness, unharmed, after ~ 6 hours. Sevoflurane is also often co-administrated with Nitric Oxide (NO) which is also in the PPARgamma pathway. The CBD dosage on the patient noted above was unknown, as this was self administered unbeknownst to the practicing physicians. This child was almost intubated, but fortunately a physician who was highly knowledgable on CBD knew this was a mistake, and it was best to let time take its course. The above paper on anesthesia highlights on page 9 the following about Sevoflurane.
Most drugs are metabolized by the liver. P450 Cytochromes are responsible for metabolism of a variety of AED’s. Some AED’s are potent inhibitors of these enzymes, and can exacerbate the side effects of another AED. Patients with variants in these p450 cytochrome genes can have altered cytochrome enzymatic activity resulting in complications with certain AEDs. Of particular notice is that CBD appears to be a potent inhibitor of CYP3A isoforms which are involved in the the metabolism of Clobazam to desmethyl Clobazam.
It is has been published that certain compounds found in some foods can influence cytochromes. Bergamottin in grapefruit juice can inhibit some cytochromes, leading to longer effective dosages of AEDs which are dependent on these same cytochromes.
Pierson-Memantine for GRIN2A related epilepsy – in ACTN 2014
Mitochondrial Connection
It was reported that CNR1 receptors are localized to the mitochondria according to Benard et al. (Benard_Mito_CB1-.3053). This was later contested by Morozov who pointed out that the antibody used in Benard’s study has cross reactivity with other mito proteins (Benard_Debunker).
Because CBD has a very low affinity for CNR1, the mode of interaction with Sevoflurane may be related to its uncontested and thoroughly reported calcium regulation in the mitochondria (seen above with Esposito et al). CBD’s interactions with PPARgamma are well published, but the suggested contra-indication with Sevofurane is speculation at this point and needs more research. The one patient which experienced this adverse event had a de novo nonsense mutation in the D4 location (nucleic acid position 4693) of the SCN1A receptor. A nonsense mutation knocks out the rest of the C-terminus protein which contains the Calmodulin binding domain (SCN1A_Catalog). Nonsense mediated decay was not reported, but CBD did limit the seizures.
Dravet Syndrome is not always limited to SCN1A mutations. 10-12% can be mutations in other genes. Here is an example of another potential cause of Dravets_LETM1.
Many researchers expressing concern over the use of cannabis extracts in children point to a few papers which attempt to measure persistent cannabis use in adolescence. The Meier paper (Adolescent Cannabis Use IQ) is the most frequently cited (although the paper has since been challenged as having poor socio-economic controls by Ole Rogeberg). This has been presented as a risk to Dravet children, but scientists voicing these concerns may not be aware of all of the papers in this space to put this risk into proper perspective.
The Meier paper demonstrates a slight decay in IQ for “cannabis use.” This is likely THC rich recreational cannabis, but the paper does not address the source. This is a major flaw in peoples’ reasoning, as it assumes all cannabis is the same. Sequence data from the genomes of cannabis and humans demonstrates cannabis plants are >10 fold more variable than humans. It is well known that compound levels (THC, CBD, etc.) in cannabis can vary dramatically. Likewise, other cannabinoids and terpenes are frequently not measured in such studies making it difficult to correlate this risk epilepsy patients using predominately CBD rich cannabis.
To put this into perspective, one might also consider the impact of AED’s, or even legal drugs like ethanol, on one’s IQ. A good functional MRI (fMRI) study on EtOH vs Cannabis demonstrates far more harm from EtOH exposure than cannabis. As for epilepsy patients, the of risk that prolonged exposure to phenobarbital (an approved AED) could alter and/or damage the development of a child’s brain is real, and should be put into perspective when thinking about the use of CBD (or even THC), which are relatively safe compounds.
The Hypocratic Oath “first, do no harm” can present physicians with errors of omission and errors of commission. The act of not doing anything (omission) in the case of Dravet Syndrome is likely to cause more harm than 6 IQ points mentioned in Meier’s paper. Likewise the long term effects of AEDs in children is an equal unknown and may be very damaging to IQ. IQ is also a debatable measure of intelligence. Much of it is memory driven, and recent papers have demonstrated that this memory deficit with THC can be mitigated with co-administration of COX-2 inhibitors like Ibuprofen. CBD is a mild COX-2 inhibitor which suggests that any co-administration of THC in the presence of CBD should work to eliminate this memory risk. Likewise, papers have recently been published demonstrating the psychoactive effects of THC can be reversed with pregnenolone administration. Compare this paper to a study of Jamaican pregnant women to add further perspective on the risk of THC (if it even exists in the CBD extracts being used for Dravet), and it is really hard to come to any grounded concern over this risk in light of what these children are facing.
This chart highlights studies measuring the Toxicity of CBD.
This is a very interesting and herculean diagnostic case (LETM1 above). After negatively Sanger sequencing all exons and splice junctions of SCN1A (est cost $2500), they graduated to a 67 gene Next Gen panel ($5500) which was also negative. They then resorted to array CGH (est cost $1500) which picked up a large (1.7Mb) deletion on chromosome 4 and an amplification on chromosome 8. Results had to be confirmed with FISH (est cost $800)
(chromosome 4)
(chromosome 8)
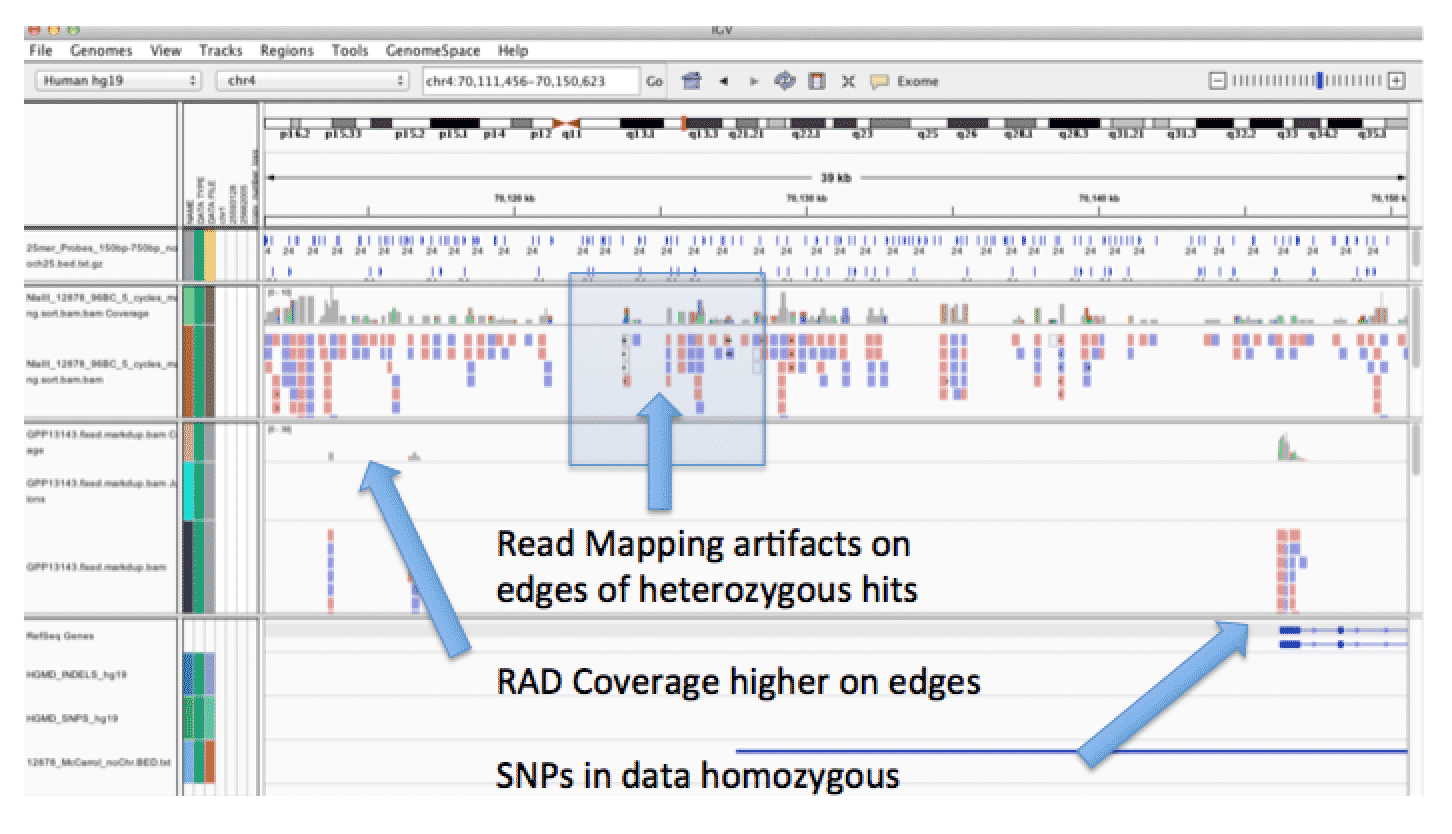
chr4 980771 981224 IDUA
chr4 981427 982004 IDUA
chr4 994280 994830 IDUA
chr4 995006 995907 IDUA
chr4 995910 996455 IDUA
chr4 996464 997013 IDUA
chr4 997020 998327 IDUA
chr8 1719139 1720021 CLN8
chr8 1728240 1728924 CLN8
References On Epilepsy Genetics
1. Lemke, J.R. et al. GRIN2B mutations in west syndrome and intellectual disability with focal epilepsy. Ann Neurol (2013).
2.Lemke, J.R. et al. Mutations in GRIN2A cause idiopathic focal epilepsy with rolandic spikes. Nat Genet 45, 1067-1072 (2013).
3. Lemke, J.R. et al. Targeted next generation sequencing as a diagnostic tool in epileptic disorders. Epilepsia 53, 1387-1398 (2012).
4. Epi, K.C. et al. De novo mutations in epileptic encephalopathies. Nature 501, 217-221 (2013).
5. Carvill, G.L. et al. Targeted resequencing in epileptic encephalopathies identifies de novo mutations in CHD2 and SYNGAP1. Nat Genet 45, 825-830 (2013).
Below are the references cited for the CBD trial ongoing with the FDA in 2014.
1. Wheless JW (2009) Managing severe epilepsy syndromes of early childhood. Journal of child neurology 24: 24S-32S; quiz 33S-26S.
2. Mechoulam R, Shani A, Edery H, Grunfeld Y (1970) Chemical basis of hashish activity. Science 169: 611-612.
3. Izzo AA, Borrelli F, Capasso R, Di Marzo V, Mechoulam R (2009) Non-psychotropic plant cannabinoids: new therapeutic opportunities from an ancient herb. Trends in pharmacological sciences 30: 515-527.
4. Zuardi AW (2008) Cannabidiol: from an inactive cannabinoid to a drug with wide spectrum of action. Revista brasileira de psiquiatria 30: 271-280.
5. Meier MH, Caspi A, Ambler A, Harrington H, Houts R, et al. (2012) Persistent cannabis users show neuropsychological decline from childhood to midlife. Proceedings of the National Academy of Sciences of the United States of America 109: E2657-2664.
6. D’Souza DC, Sewell RA, Ranganathan M (2009) Cannabis and psychosis/schizophrenia: human studies. European archives of psychiatry and clinical neuroscience 259: 413-431.
7. Karler R, Turkanis SA (1980) Subacute cannabinoid treatment: anticonvulsant activity and withdrawal excitability in mice. British journal of pharmacology 68: 479-484.
8. Izquierdo I, Orsingher OA, Berardi AC (1973) Effect of cannabidiol and of other cannabis sativa compounds on hippocampal seizure discharges. Psychopharmacologia 28: 95-102.
9. Izquierdo I, Tannhauser M (1973) Letter: The effect of cannabidiol on maximal electroshock seizures in rats. The Journal of pharmacy and pharmacology 25: 916-917.
10. Cox B, ten Ham M, Loskota WJ, Lomax P (1975) The anticonvulsant activity of cannabinoids in seizure sensitive gerbils. Proceedings of the Western Pharmacology Society 18: 154-157.
11. Consroe P, Benedito MA, Leite JR, Carlini EA, Mechoulam R (1982) Effects of cannabidiol on behavioral seizures caused by convulsant drugs or current in mice. European journal of pharmacology 83: 293-298.
12. Jones NA, Hill AJ, Smith I, Bevan SA, Williams CM, et al. (2010) Cannabidiol displays antiepileptiform and antiseizure properties in vitro and in vivo. The Journal of pharmacology and experimental therapeutics 332: 569-577.
13. Jones NA, Glyn SE, Akiyama S, Hill TD, Hill AJ, et al. (2012) Cannabidiol exerts anti-convulsant effects in animal models of temporal lobe and partial seizures. Seizure : the journal of the British Epilepsy Association 21: 344-352.
14. Mechoulam R, Carlini EA (1978) Toward drugs derived from cannabis. Die Naturwissenschaften 65: 174-179.
15. Cunha JM, Carlini EA, Pereira AE, Ramos OL, Pimentel C, et al. (1980) Chronic administration of cannabidiol to healthy volunteers and epileptic patients. Pharmacology 21: 175-185.
16. Ames FR, Cridland S (1986) Anticonvulsant effect of cannabidiol. South African medical journal = Suid-Afrikaanse tydskrif vir geneeskunde 69: 14.
17. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, et al. (2009) Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of biomedical informatics 42: 377-381.
18. Chiron C, Marchand MC, Tran A, Rey E, d’Athis P, et al. (2000) Stiripentol in severe myoclonic epilepsy in infancy: a randomised placebo-controlled syndrome-dedicated trial. STICLO study group. Lancet 356: 1638-1642.
19. Wheless JW (2006) Intractable epilepsy: A survey of patients and caregivers. Epilepsy & behavior : E&B 8: 756-764.
20. Bourgeois BF (2013) Initiating antiepileptic drug treatment and characteristics of drugs. Handbook of clinical neurology 111: 719-725.
21. Consroe P, Martin P, Eisenstein D (1977) Anticonvulsant drug antagonism of delta9tetrahydrocannabinol-induced seizures in rabbits. Research communications in chemical pathology and pharmacology 16: 1-13.
22. Turkanis SA, Karler R (1981) Excitatory and depressant effects of delta 9-tetrahydrocannabinol and cannabidiol on cortical evoked responses in the conscious rat. Psychopharmacology 75: 294-298.
©2025 MEDICINAL GENOMICS CORPS
Copyright all right reserved.
Signup to receive email updates on blog posts, podcasts, webinars, new products, and more!
Join us at the CannMed 25 Innovation and Investment Summit, an exclusive experience of discovery, development, and networking with innovators from around the world.