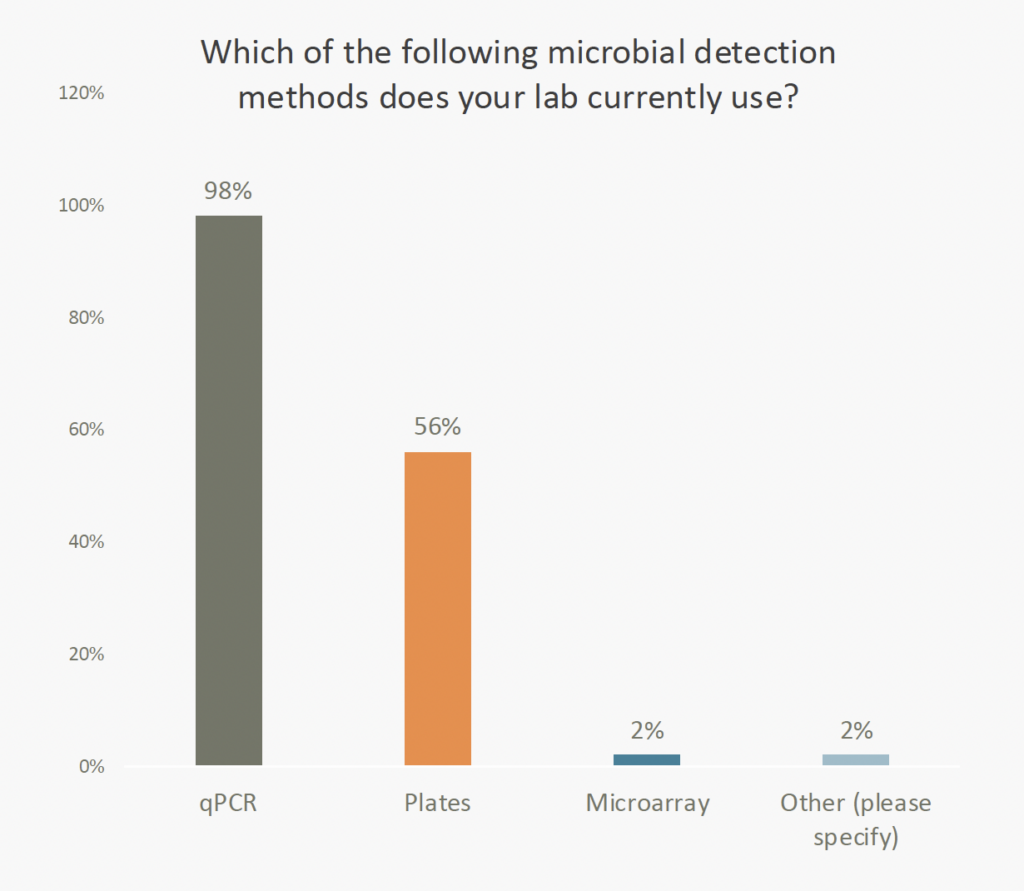
A new study found that 98% of cannabis labs surveyed use qPCR for microbial detection, making it the “gold standard” for microbial safety testing in the industry.
These results indicate a decisive migration away from traditional plating techniques, which were the preferred technology in the early days of cannabis safety testing. As the industry matures, more cannabis labs have come to recognize the speed, sensitivity, and specificity of qPCR testing. The shift mirrors broader trends within leading health institutions like the CDC and FDA, which increasingly depend on molecular methods for precise pathogen detection and outbreak management.
About the study
Medicinal Genomics, in partnership with Baccus Research, conducted a nationwide survey of cannabis testing laboratories, unveiling crucial insights shaping the future of the cannabis industry, while identifying emerging trends and potential shifts in testing methodologies, regulatory frameworks, and industry practices.
To ensure the study’s findings were grounded in real-world industry experience, researchers targeted respondents who played an active role in decision-making related to microbial detection testing, such as CEO, COO, Lab Director, Compliance Officer, Testing Scientist, or Microbiologist.
A dual approach
Despite qPCR’s dominance in cannabis microbial testing, 56% of labs continue to use plating technology alongside molecular techniques. Labs maintain this dual approach because of historical precedent, legacy systems, or compliance with specific regulatory requirements. However, the inherent drawbacks of plating methods—such as time-intensive incubation, reduced sensitivity, and challenges in identifying viable but non-culturable organisms—are accelerating the adoption of molecular solutions. Laboratories are recognizing that qPCR not only meets but exceeds the demands of modern microbial detection, offering real-time data that is critical for swift decision-making.
A foundation for the future
The broader implications of this shift to qPCR solutions are transformative. Molecular diagnostics like qPCR empower laboratories to enhance efficiency, improve data accuracy, and align with the increasingly stringent expectations of regulatory bodies. For instance, the CDC’s reliance on molecular assays for foodborne pathogen surveillance and the FDA’s adoption of qPCR in environmental monitoring demonstrate the scalability and reliability of these methods. These agencies’ integration of molecular technologies sets a clear precedent for laboratories to follow, solidifying qPCR as the emerging gold standard.
Furthermore, molecular diagnostics are not only a response to current industry needs but also a proactive investment in the future. As laboratories adopt qPCR, they position themselves to meet evolving regulatory landscapes, address public health challenges more effectively, and remain competitive in a rapidly advancing field.
Future outlook
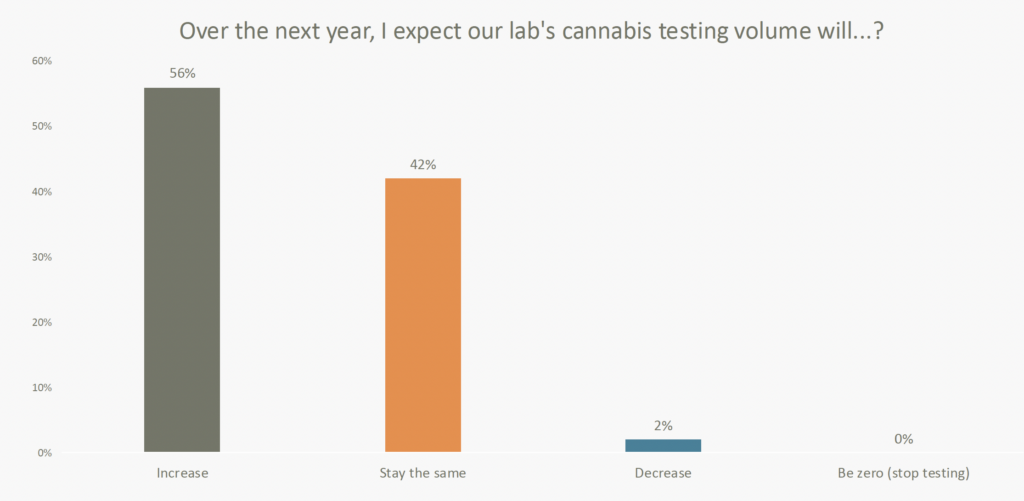
Speaking of the future, 56% of labs surveyed expect an increase in cannabis testing over the next year, reflecting sustained demand and a promising market outlook. As regulatory frameworks evolve and the cannabis industry expands, testing requirements will increase, driving innovation and creating opportunities for labs to enhance their methodologies.
Growth opportunities
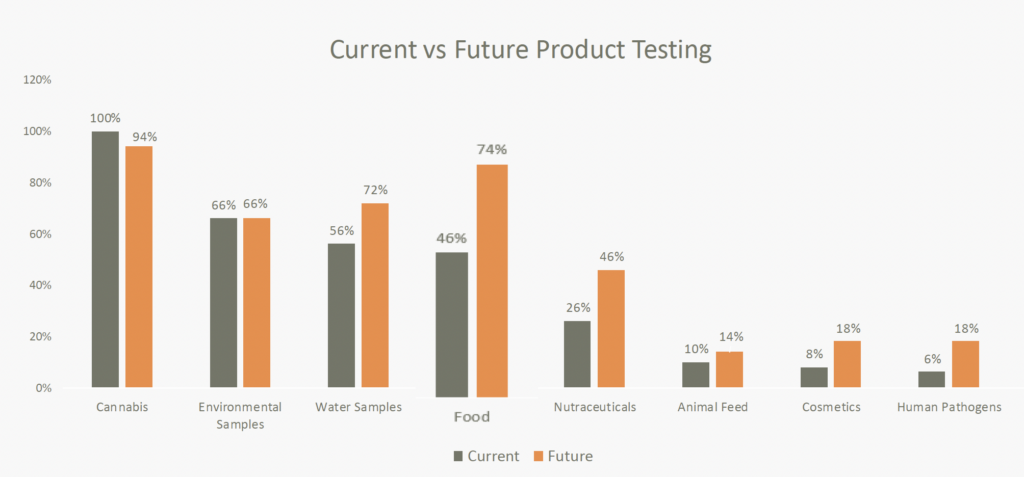
Labs also expect to expand beyond cannabis testing. Currently, 46% of labs surveyed test food products, with 74% planning to expand into this area in the coming years. Similarly, 56% test water samples, and 72% plan to do so in the near future. As labs plan for expansion, selecting a provider that can deliver solutions for all sectors will be crucial.
Incorporating genomic services
Another key trend is the growing adoption of genetic testing, with 54% of labs already integrating genomics into their workflows. This trend underscores the value genetic insights bring to quality assurance, product development, and safety protocols. In cannabis testing, genetic testing is particularly crucial for strain verification, potency profiling, and pathogen resistance analysis—important for compliance and innovation.
The intersection of cannabis and genetic testing is especially exciting as it aligns with broader scientific and regulatory trends. Genomic applications are increasingly central to improving product consistency, yield, and addressing public health concerns. As more labs embrace genetic testing, they will not only lead in scientific rigor but also pave the way for integrating advanced technologies like next-generation sequencing (NGS).
Evaluating qPCR vendors
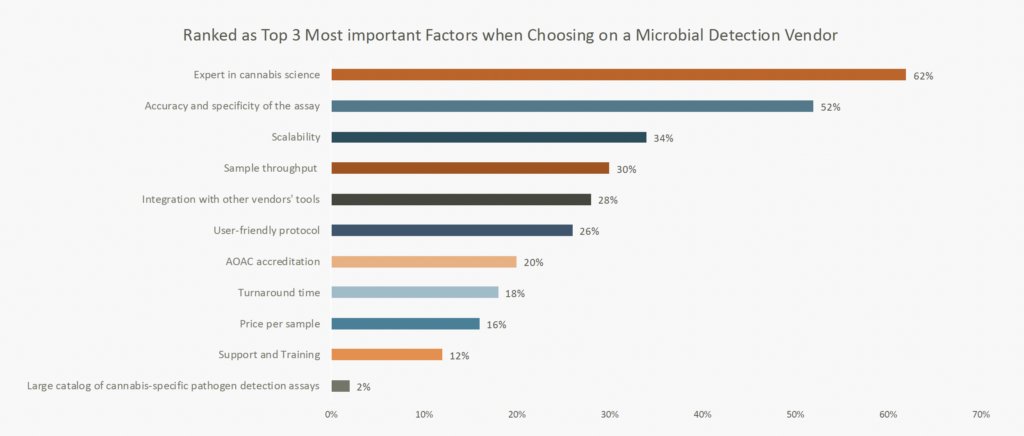
The study uncovered several key factors driving the selection of microbial detection vendors. While expertise in cannabis science remained a priority, accuracy and specificity of assays, along with scalability and sample throughput, emerged as the top considerations. Notably, vendors that offer “microbial target testing using qPCR specifically designed for cannabis and hemp” were identified as having the most compelling advantage.
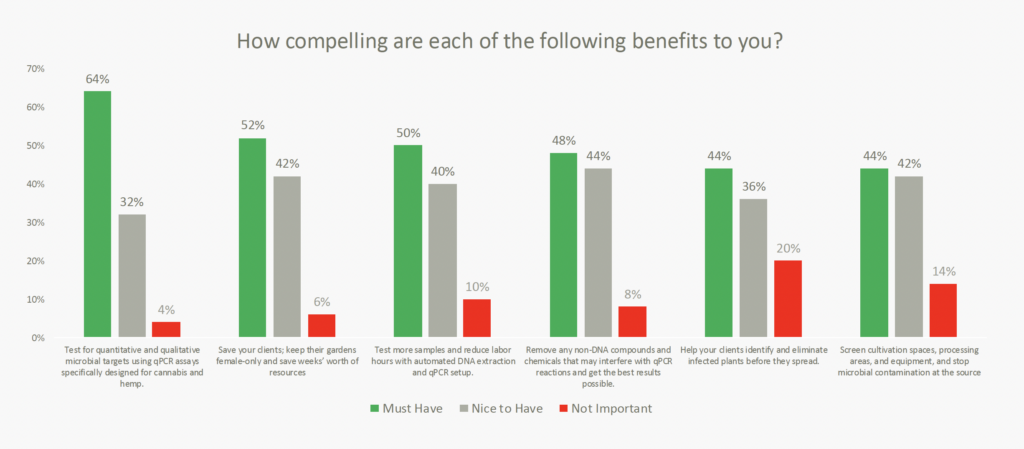
The findings also reaffirmed Medicinal Genomics’ strong reputation as an industry leader, emphasizing our exceptional service and support, highly effective sales and marketing team, and the superior quality of our assays. These strengths underscore our commitment to delivering value to our customers and maintaining our leadership in the microbial testing sector.
Lessons learned
The survey has highlighted valuable opportunities for Medicinal Genomics to improve its offerings, specifically in the areas of AOAC accreditation, sustainability, expansion into other verticals, and ease of use.
AOAC certification
To enhance our product offering and better serve the industry, we are working to streamline our PathoSEEK assays and complete AOAC certifications for assays that currently lack cannabis-specific Standard Method Performance Requirements (SMPRs). This process includes validating Total Aerobic Count (TAC), Coliforms, Bile-Tolerant Gram-Negative (BGTN) bacteria, and Listeria in food matrices. Once these certifications are achieved, we will shift our focus back to extending these assays to additional cannabis-specific matrices, ensuring a broader and more versatile application.
Sustainability
We are also excited to introduce lyophilization as part of our next-generation solutions. This freeze-drying process significantly reduces the weight and volume of our products, lowering shipping and logistics costs while simultaneously minimizing our environmental footprint. By offering this option, we help our clients adopt greener practices while saving money, aligning with the industry’s growing emphasis on sustainability.
Expansion into Other Verticals
Our firm is also expanding its validation efforts and product portfolio to include other agricultural crops, such as mushrooms. We’ve recently applied our plant pathogen assays to strawberries and lettuce, further demonstrating the adaptability of our solutions to meet emerging needs in agriculture.
Ease of Use
Additionally, we are thrilled to announce the design and launch of the next-generation PathoSEEK assay, featuring MaGiC Lysis, a streamlined DNA extraction process. This groundbreaking enhancement directly addresses longstanding lab concerns about ease of use and sample throughput, making the process more user-friendly and efficient.
Our Commitment to Innovation
Our commitment to listening to industry feedback remains steadfast. These findings serve as both validation of our efforts and a roadmap for continued growth. By addressing current challenges, implementing innovative solutions like lyophilization, and enhancing assay technology, we ensure our offerings continue to meet and exceed the evolving needs of the cannabis testing industry.








