Cannabis testing labs nationwide use culture-based methods to test cannabis for harmful microbes, such as E.coli, salmonella, yeast and mold, and other bacteria. The most popular culture-based test is petrifilm. However, Petrifilm has never been validated for use on cannabis, which has a diverse and complex microbiome.
At Medicinal Genomics, we have invested time and resources into validating our SenSATIVAx® DNA purification method and PathoSEEK® qPCR Microbial Detection Platform on cannabis flower, extracts, and a variety of infused products, so testing labs, cultivators, manufacturers, and consumers can trust the results.
We have also published peer-reviewed studies that show culture-based testing methods, including Petrifilm, inaccurately measure microbial contamination on cannabis flower and do a poor job detecting Aspergillus, which is a pathogen that has been linked to the death of more than one cannabis patient.
We discovered similar limitations in culture-based testing while developing our new qPCR-based method for microbial detection on cannabis extracts and marijuana-infused products (MIPS). Namely, petrifilm also does a very poor job of detecting E.coli contamination in oil and candies, and it’s likely to pass oils or candies that are contaminated with E.coli.
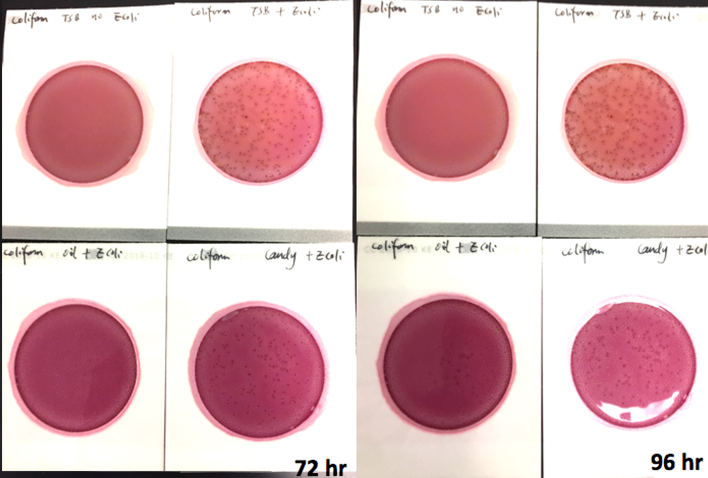
We spiked a known amount of live E.coli into three different environments: tryptic soy broth (TSB), hemp oil, and hard candy. We then homogenized the samples, pipetted amount from each onto Petrifilm E. coli / Coliform Count (EC) Plates, and incubated for 96 hours. We also placed TSB without any E.coli onto a petrifilm to serve as a control. The images below show the results in 24-hour intervals.
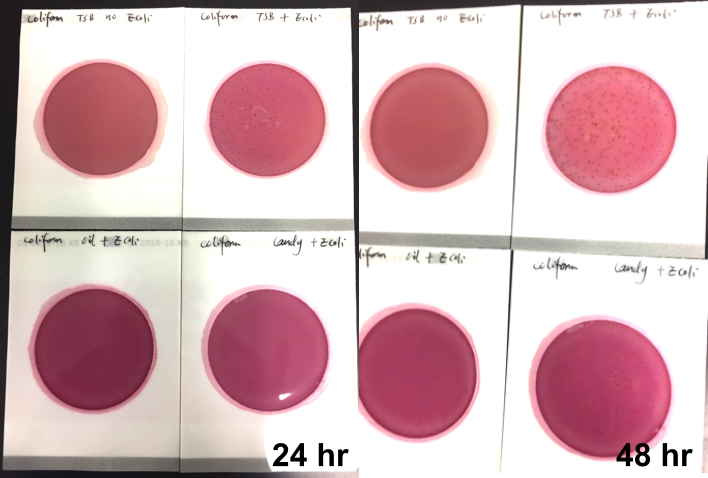
After 24 hours:
The results after 24 hours are important because the manufacturer suggests a 24-hour incubation for its E. coli / Coliform Count test. That means, if labs were to follow the manufacturer’s instructions, they would pass samples that are contaminated with viable E.Coli.
After 48 hours, colonies start to form on candy plate, and it continues to grow over the next two days. The oil plate on begins to show slight growth at the 96-hour mark. This indicates the E.coli is alive and growing in both samples. It’s just slower than the test can detect.
We extracted DNA from the same spiked samples that were used on the petrifilm using the SenSATIVAx DNA purification method at two time periods. Once immediately after the spike and another after 24 hours of incubation. We then used The PathoSEEK E. coli and Salmonella qPCR detection assay to measure the amount of E.Coli DNA present in the environments.
The qPCR results show that E.coli is viable and growing in the oil and candy environments. In fact, it is growing more than the tests would indicate, particularly in the oil.
| E.coli in TSB | E.coli in Oil | E.coli in Candy | |
| Initial | 33.13 | 28.9 | 30.71 |
| After 24 hours | 22.56 | 27.11 | 25 |
| Δ Cq | 10.57 | 1.79 | 5.71 |
| Fold growth | 1520 | 3.5 | 52 |
It’s unclear why the petrifilm does not show the same rate of growth as the qPCR method. Perhaps there is something in the candy and oil that affects the plate’s readout chemistry. Further research may determine the cause.
Nevertheless, the results illustrate the fundamental problem culture-based microbial testing methods to test cannabis samples. Culture-based methods have not been validated on cannabis or cannabis-derived products, so their results cannot be trusted.
We invite testing labs to review our SenSATIVAx® and PathoSEEK® Manufacturer Validation document.
©2024 MEDICINAL GENOMICS CORPS
Copyright all right reserved.
Signup to receive email updates on blog posts, podcasts, webinars, new products, and more!
Join us at the CannMed 24 Innovation and Investment Summit, an exclusive experience of discovery, development, and networking with innovators from around the world.