In recent weeks we have witnessed plant pathogen test providers advertise the extreme sensitivity of their hop latent viroid (HLVd) testing kits. While we agree that the numbers are impressive, growers must remember to use this tool with caution. If they are not careful, that sensitivity can do more harm than good.
Setting a threshold for infectiousness
Let’s first acknowledge that there are a lot of unknowns when it comes to HLVd. For example, we do not know how many HLVd copies must be present in order for a plant to be infectious. It’s possible, and likely, that sensitive qPCR assays will detect low levels of HLVd RNA in resistant plants that have recently been exposed to the viroid. However, if growers deploy a policy where they eliminate all plants that show any signal on a highly sensitive qPCR assay, they may end up tossing healthy, resistant plants!
Cycle thresholds
We saw a similar problem in the early days of qPCR testing for COVID-19. Often, people were determined “medical cases” based on qPCR results that showed very low levels of virus RNA. Some tests were using cycle threshold (Ct) scores over 40. As we learned more about the virus, the COVID PCR world set a more reasonable Ct threshold for determining infectious patients. Rita Jaafar published a paper performing this calibration of PFU (plaque forming units) to Ct scores from qRT-PCR. They found that only samples with a Ct lower than 35 will culture. Samples with Cts greater than 35 mostly contained viral fragments (Dead RNA) that were also difficult to sequence to prove they were not false positives. Unfortunately, this culture-based calibration cannot be replicated with HLVd because no one has published a method to culture the viroid.
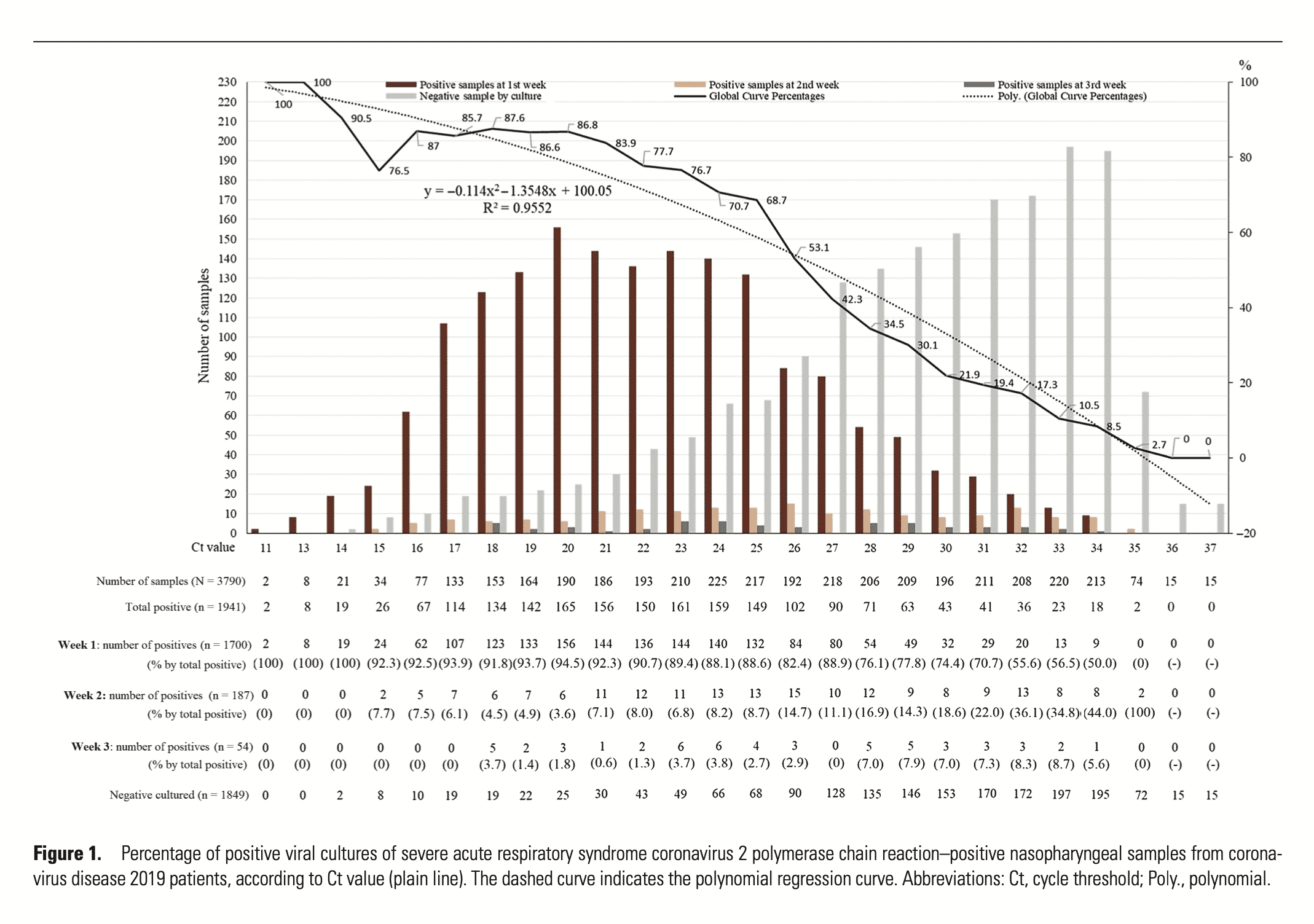
Similarly, Rabaan et al found that Ct scores over 36 for RNA viruses are of no value (non-diagnostic result). In fact, they argue quarantining people with high Ct results and low viral loads is harmful because those people are likely to be already immune and you do not want to quarantine your immune population as they are needed for herd immunity.
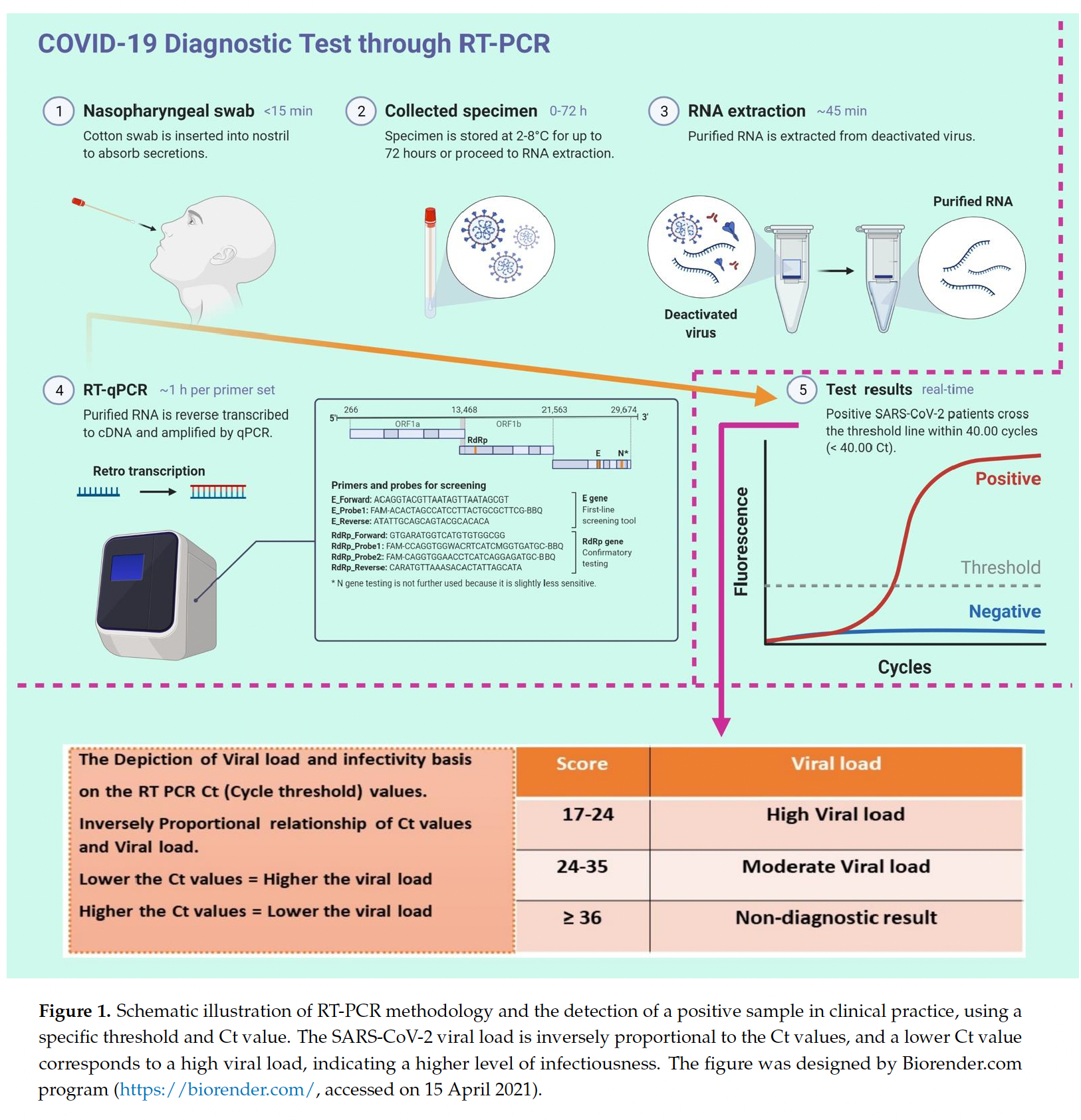
Lastly, most COVID-19 testing sites will now only sequence validate samples with Ct scores less than 32. The CDC has even stricter limits, only sequencing samples with Ct scores less than 28.

Copy Numbers
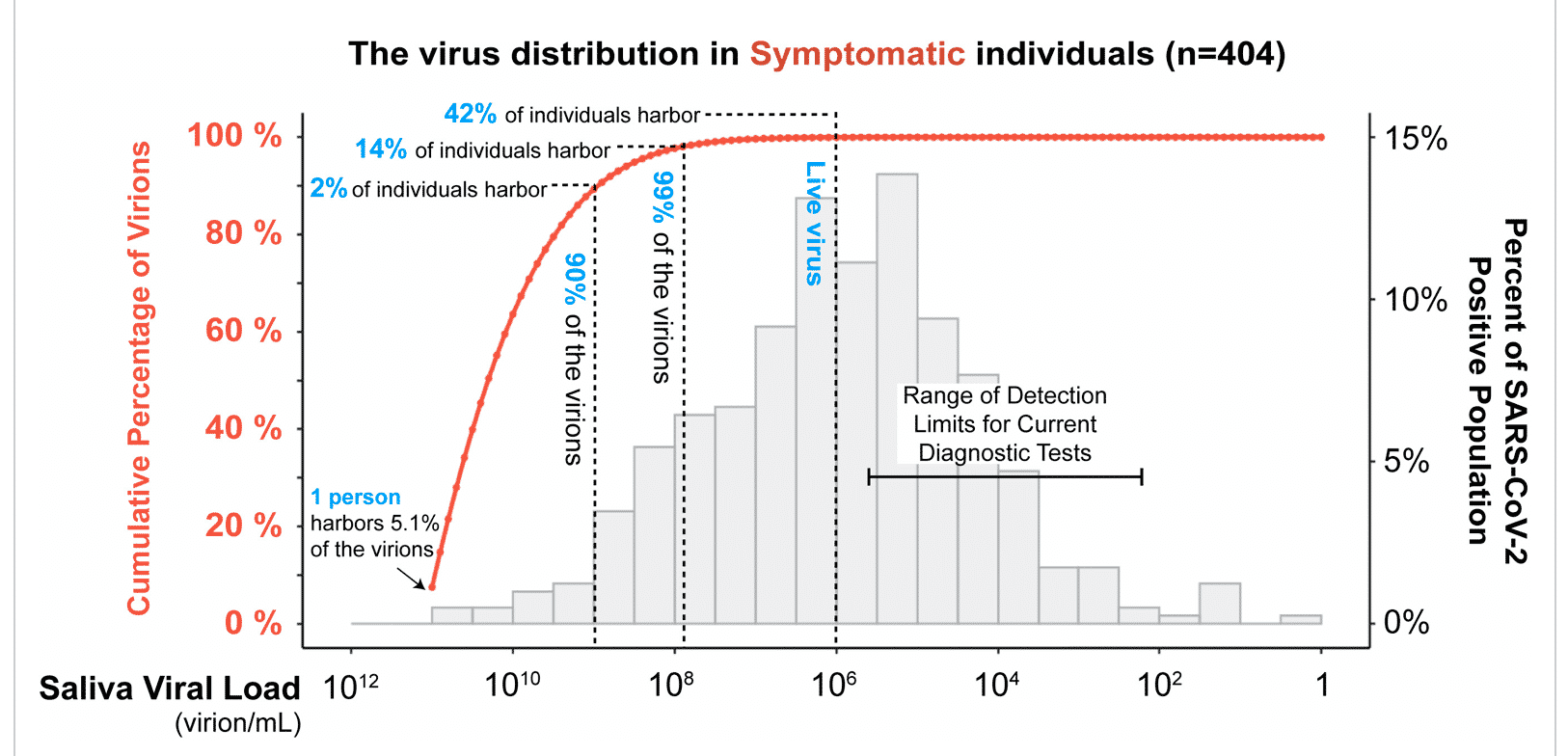
Another perspective is the graph below from Yang et al, which displays the data in terms of virus copy numbers. You will note that the companies chasing 4 copies of HLVd would be on the far right side of this graph, several orders of magnitude below the detection limits of diagnostic tests. Safe to assume that cranking your detection limits beyond that which is acceptable for most diagnostic tests, like COVID testing, is likely to suggest culling the very plants you want to keep.
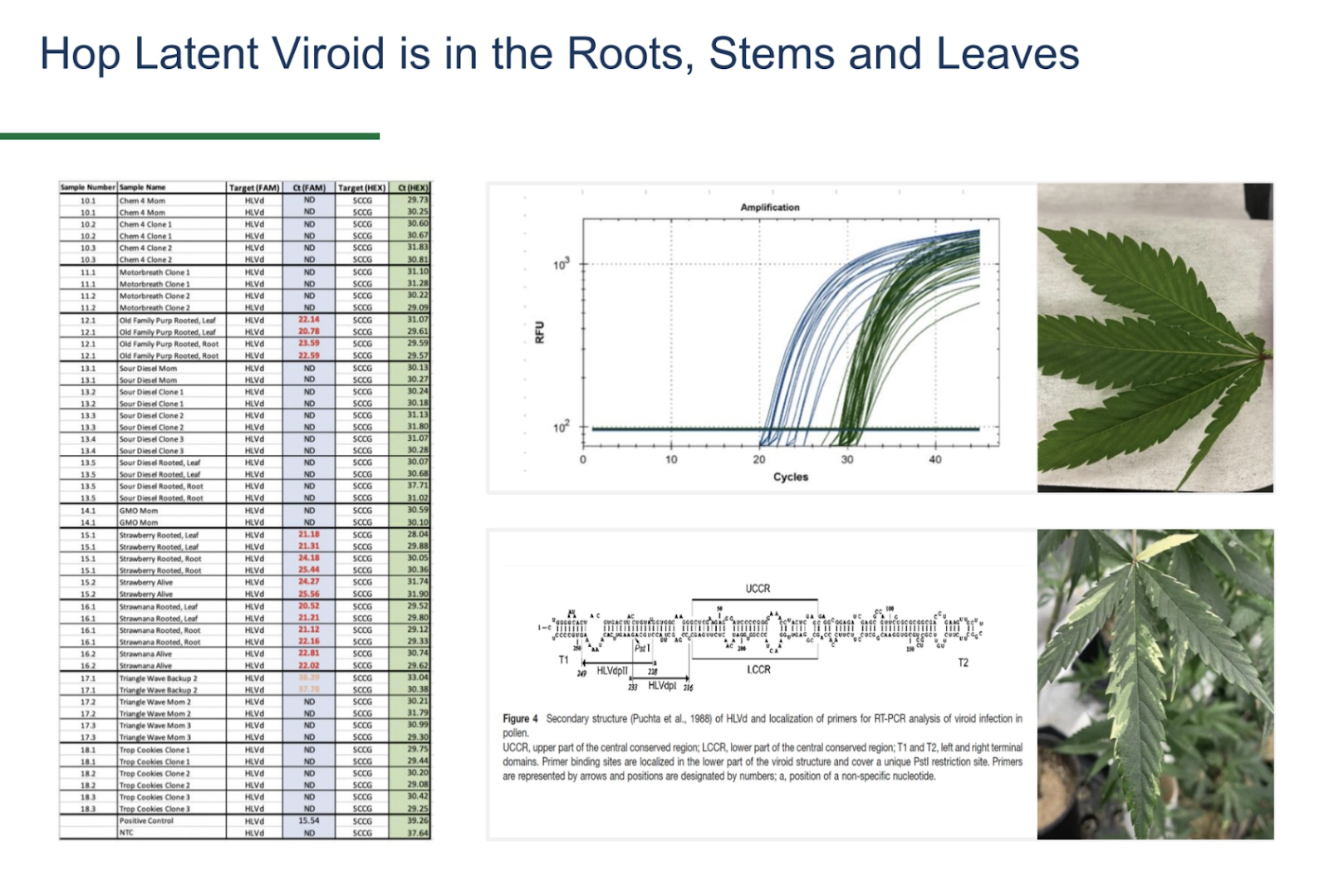
The current protocol for our PathoSEEK Cannabis Virus Multiplex A is set to 40 cycles (like most qPCR virus protocols including those for COVID-19) to ensure that we are capturing well past the diagnostic threshold. However, the HLVd-positive plants we identified delivered high viroid loads with Ct scores in the range of 17 to 30.
Making a case for caution
HLVd is a latent viroid, and it’s still not clear if it causes reduced yield and dudding in all cultivars. It may be that the plants that remain asymptomatic and/or have low viroid loads may be the plants you want the most. The reality is we still at a very early stage of screening for this viroid and know little about the Ct values that are most productive for screening a population of diverse cannabis genetics.
Plants with Ct scores greater than 33 should be tracked carefully. Do not discard any valuable mother plants from a single late Ct qPCR result. Isolate the questionable plant, and repeat the test the following day. Create a log of how many times Ct scores are reproducible upon repeat testing. This will train your lab on the trustworthy Ct range. Aiming for single-molecule detection without this level of confirmation will lead to many false positives. If you are a grower who outsources testing to a lab, ask for the Ct scores so you can train your facility on the Ct scores predictiveness over time. This is one reason we have been skeptical of simple qualitative tests for HLVd.
This is not an argument against high sensitivity tests. It is an argument against assuming that the biology of HLVd viroid load is binary. The very existence of the viroid may not predict whether a plant will succumb to the disease. High viroid loads are a safe bet to cull or isolate but very low viroid loads may be a signature of the most resistant plants. Repeat testing across time is the most prudent approach.








