Last week, The Bureau of Cannabis Control released its long-awaited cannabis regulations. The document details the regulations for all aspects of the cannabis industry from cultivation to retail.
At Medicinal Genomics, we are particularly interested in the microbial testing requirements, as it affects the laboratories in California that are using our PathoSEEK® Microbial Safety Testing Platform.
All in all, our laboratory customers will be happy with the regulations. They are favorable to qPCR methods like PathoSEEK because the state requires testing for specific microbial targets and does not set detection thresholds using colony forming units (CFU).
Microbial Targets
California is the first state to require testing for only pathogenic microbial impurities:
- Shiga toxin-producing E. coli (STEC)
- Salmonella
- Pathogenic Aspergillus species (A. fumigatus, A. flavus, A. niger, and A. terreus)
The state requires cannabis testing labs test inhaled cannabis products for all six targets, while non-inhaled products need only be tested for STEC and Salmonella.
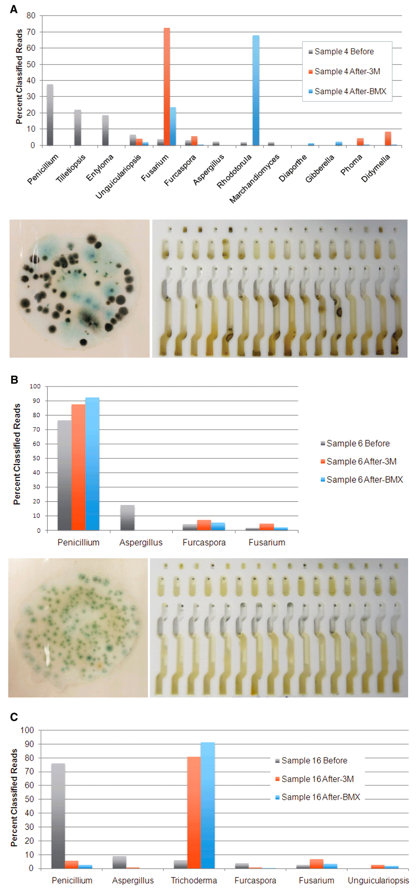
Cannabis testing labs that are currently using culture-based tests for microbials will have a very difficult time validating their method on Aspergillus. Aspergillus is notoriously difficult to grow in a culture medium because it tends to grow very slowly and colonies clump together to form a heterogenous macrocolony (see below). As a result, there are no commercially available culture-based tests that can detect Aspergillus.

However, qPCR methods can detect Aspergillus DNA. Our PathoSEEK Aspergillus detection assays have been validated on cannabis flower, extract, and edibles. We have also multiplexed all four Aspergillus species into a single assay, so testing labs only need to use one well on a 96-well plate.
Detection Threshold
All six targets that California requires are presence/absence tests. If any of the target organisms is detected within a 1g sample, the entire batch fails and can’t be released for retail sale. A failed batch can be remediated if it fails microbial testing, but it must be resubmitted and pass all laboratory testing before it can be released for retail sale. A batch can be remediated twice.
The language used in the regulations is significant because it does not use the term CFU, which has been used in other state regulations to set detection thresholds. CFU is a unit of measure used to quantify the results of a culture based tests. However, since qPCR methods measure microbial contamination using DNA, results are given in Cq (quantification cycle), not CFU.
We have developed equations to convert Cq to CFU for states that use that unit of measure, but it has always felt like fitting a square peg into a round hole because the two values are measuring different things. qPCR measures the amount of DNA present in a sample, while CFU measures the number of colonies that grew in a culture medium after a period of time. We have demonstrated in peer-reviewed papers that the microbes present on a cannabis sample and the microbes that grow in a culture medium can be radically different (see image below). More importantly, the latter is not an accurate representation of the potential harm to cannabis users and patients.
Complete Solution
At Medicinal Genomics, we have developed qPCR testing assays for all six of the required targets. We have multiplexed STEC and Salmonella into one assay and all four Aspergillus into another single assay. That means labs can test for all six microbial targets in two wells (not including positive and negative controls).
We have also documented our validation on cannabis flower, extract, and edibles, which is publically available for download on our Validation Documents page. No other microbial testing platform has gone through such a thorough validation.
If you are a cannabis testing lab in California, contact us today to learn why the PathoSEEK platform is your best choice for microbial testing. Or if you prefer, you can review our validation data and protocols, and then order directly from our webstore.








